|

Predicting the sign of the free energy change
A test case
Suppose that the task is predicting the protein stability change when
Glu (E) 200 is mutated into Lys (K) in the Prion Protein (PDB: 1HJM
chain A) at pH 7 and Temperature equal to 5 °C (Fig.2).
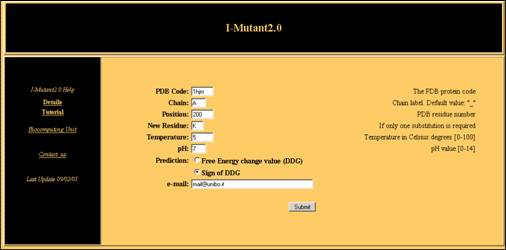
Input
The following operations are required:
-
Fill in the PDB Code
box with the PDB code of your protein.
-
In Chain box,
put the chain label of the protein under study, if necessary.
-
In the Position
box insert the PDB number of the residue that undergoes mutation.
- In New Residue insert
the required one letter code mutation (optional).
-
The boxes pH
and Temperature can be filled with the experimental
conditions at which the experiment is simulated.
The selection of the
radio button “Sign of DDG” allows the prediction of
the sign of
the free energy change value. The output of the predictor will be send
to your e-mail to address, if required, or shown directly to you in due
time.
Output
The results obtained for the given test case are listed below:
PDB File: /junk/I-Mutant/Mut11370/pdb1hjm.ent Chain: A
Position WT NEW Stability RI pH T RSA
200 E V Decrease 0 7.0 5 89.3
200 E L Decrease 1 7.0 5 89.3
200 E I Decrease 4 7.0 5 89.3
200 E M Increase 1 7.0 5 89.3
200 E F Increase 1 7.0 5 89.3
200 E W Decrease 2 7.0 5 89.3
200 E Y Decrease 1 7.0 5 89.3
200 E G Decrease 7 7.0 5 89.3
200 E A Decrease 5 7.0 5 89.3
200 E P Increase 0 7.0 5 89.3
200 E S Decrease 5 7.0 5 89.3
200 E T Decrease 3 7.0 5 89.3
200 E C Decrease 3 7.0 5 89.3
200 E H Decrease 6 7.0 5 89.3
200 E R Decrease 2 7.0 5 89.3
200 E K Decrease 7 7.0 5 89.3
200 E Q Decrease 4 7.0 5 89.3
200 E N Decrease 4 7.0 5 89.3
200 E D Decrease 2 7.0 5 89.3
WT: Aminoacid in Wild-Type Protein
NEW: New Aminoacid after Mutation
RI: Reliability Index
T: Temperature in Celsius degrees
pH: -log[H+]
RSA: Relative Solvent Accessible Area
If you do not ask for a specific
mutation in the
position at hand, all the possible mutations will be taken into
consideration, including the one that may be of specific interest.
However if you are asking only one mutation, by activating the "New
Residue" option, one prediction will be returned. The free energy
change corresponding to the mutation of Glu (E) 200 to Lys (K) in 1HJM
chain A at temperature of 5 Celsius degree and pH 7 decreases,
predicting a destabilisation of the protein upon mutation.The value of
reliability index (RI) is 7 (0<RI<10). The RSA column lists
the relative solvent
accessible surface calculated with the DSSP program [2].
[1] Berman HM, Westbrook J, Feng Z,
Gilliland G, Bhat TN, Weissig H, Shindyalov IN, Bourne PE (2000). The
Protein Data Bank. Nucleic Acids Res. 28, 235-242.
[2] Kabsch W, Sander C (1983). Dictionary of protein secondary
structure: pattern of hydrogen-bonded and geometrical features. Biopolymers.
22, 2577-2637.
|